For decades we assumed that ageing was a linear, cumulative and inevitable process. Today, biology is challenging that paradigm. Epigenetics - the system of chemical marks that regulates which genes are turned on or silenced - has become one of the most promising territories in longevity medicine.
What once seemed like science fiction is now entering the clinical phase: partial epigenetic reprogramming already being tested in humans.
The origin of the concept: resetting without erasing identity
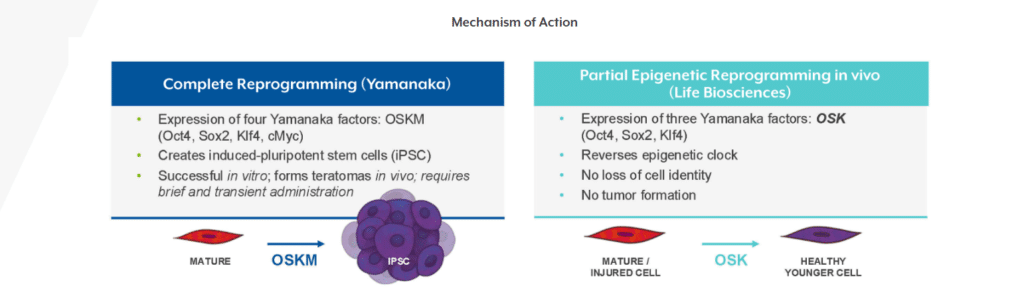
Cell reprogramming became world-renowned with the discovery of Yamanaka's factors (OCT4, SOX2, KLF4 and c-MYC), which are capable of converting adult cells into induced pluripotent stem cells (iPSCs). This process erases cell identity and resets the biological state to an embryonic stage.
The problem: complete reprogramming implies loss of specialised function and risk of tumourigenicity.
The solution that has emerged in the last decade is the partial reprogrammingTemporary activation of some factors (usually OCT4, SOX2 and KLF4, without c-MYC) to rejuvenate the epigenome without de-differentiating the cell.
In animal models, this strategy has:
- Vision restored in mice with optic nerve damage
- Improved tissue function in ageing tissues
- Reduced epigenetic age measured by molecular clocks
- Increased health parameters in accelerated ageing models
The key finding is that youth information appears to be conserved in the epigenome, and can be reactivated.
In 2026, the FDA authorised the start of the first human clinical trial based on this technology. The treatment is called ER-100, developed by Life Biosciences.
This phase 1 study evaluates safety and preliminary efficacy signals in patients with
- Open-angle glaucoma
- Non-arteritic anterior ischaemic optic neuropathy (NAION)
Both pathologies involve degeneration of retinal ganglion cells and progressive damage to the optic nerve.
What exactly does ER-100 consist of?
ER-100 is a locally administered intraocular gene therapy which uses an adeno-associated viral vector (AAV) to introduce a controlled set of reprogramming factors into cells:
OCT4, SOX2 and KLF4 (OSK)
These factors do not modify the DNA itself, but reorganise epigenetic marks (DNA methylation, chromatin remodelling, histone modifications), restoring more youthful gene expression patterns.
Critical differential element: temporal control
One of the most sophisticated aspects of the design is that the expression of the factors is under a inducible system, activatable by doxycycline. This allows:
- Activate reprogramming for a limited period of time
- Stop if adverse effects occur
- Minimising the risk of dedifferentiation
This temporal control is one of the main differences from previous attempts at more aggressive reprogramming.
Why start with the eye?
The choice is not accidental.
The ocular environment offers strategic advantages:
- It is a relatively isolated compartment (less systemic exposure).
- Enables accurate local administration.
- Visual function is measurable with objective biomarkers.
- There is strong preclinical data in animal models.
In non-human primates, OSK expression restored juvenile epigenetic patterns and improved neuronal function after optic damage.
The eye functions here as controlled test model to validate biosafety before considering systemic applications.
How does ER-100 differ from other longevity approaches?
Most current longevity interventions address the consequences of ageing:
- Senolytics → eliminate senescent cells
- Metformin or rapamycin → modulate metabolic pathways
- NAD+ boosters → support mitochondrial metabolism
- Hormone therapies → restore declining levels
ER-100 is conceptually different:
does not modulate the damage, it attempts to restore the original cellular programming.
It is an upstream intervention on the epigenetic architecture that regulates multiple biological processes simultaneously.
This places it in a potentially transformative category.

Clinical potential if results are positive
If safety and functional efficacy are demonstrated, the implications would be profound:
Neurodegeneration
It could open a pathway for diseases such as Parkinson's or Alzheimer's, where epigenetic dysfunction contributes to neuronal loss.
2. Tissue regeneration
Restore function in tissues with low regenerative capacity (heart, retina, nervous system).
Preventive medicine of ageing
Rejuvenate epigenetic profiles before overt pathology appears.
4. Biomarkers of biological age
Validate epigenetic clocks as clinical tools to measure actual reversal of ageing.
We are talking about intervening on the common denominator of multiple age-related diseases.
Risks and scientific prudence
Enthusiasm must be balanced with caution.
Potential risks include:
- Oncogenic activation
- Epigenetic instability
- Immune response to the viral vector
- Long-term unintended effects
Phase 1 is focused on safety. It does not seek to demonstrate systemic rejuvenation, but to establish that the procedure is feasible in humans.
A paradigm shift in health and wellbeing
From the perspective of well-being and longevity, this trial marks a turning point. For the first time, the hypothesis that ageing is biologically reversible is being evaluated under formal regulatory oversight.
This is not a supplement, nor is it a molecular cosmetic intervention. We are looking at the possibility that the epigenome - that dynamic system that organises our biological identity - can be reprogrammed in a controlled way.
If safety is confirmed and positive functional signals appear, the impact could redefine 21st century medicine: from treating individual diseases to restoring the functional youthfulness of tissues.
The biological clock no longer seems so immutable.






