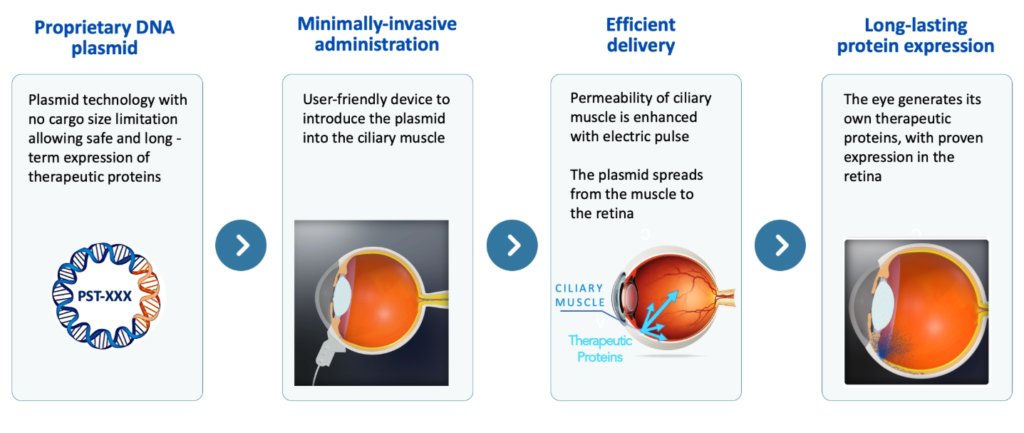
The French biotech company PulseSight Therapeutics announced the start of the first human clinical trial of its innovative non-viral gene therapy, PST-611, aimed at treating the dry form of age-related macular degeneration (AMD) and its progression to geographic atrophy (GA). The first patient has already received the initial dose, giving the green light to a Phase I study that will assess the safety and tolerability of the treatment.
Why is this therapy so important?
- Dry AMD is the leading cause of painless central vision loss in the elderly. In its advanced stage, geographic atrophy causes irreversible damage to the retina, severely impairing the patient's quality of life.
- So far, existing treatments, especially for the wet form of the disease, require frequent intraocular injections, with limited benefit for the dry form.

Mechanism of PST-611: regulating iron in the retina
- PST-611 is a first-of-its-kind therapy that does not use viruses as a vector, but an electro-transfection technology applied to the ciliary muscle. In this way, it introduces DNA encoding human transferrin, a key protein in iron regulation.
- Excess iron in the retina leads to inflammation, oxidative stress and cell death (ferroptosis), central processes in dry AMD and GA.
- Minimally invasive delivery into the ciliary muscle, avoiding frequent retinal injections.
- Muscle cells act as "biofactories" that continuously produce transferrin.
- Phase I: single dose-escalated study in 6-12 patients with dry AMD or geographic atrophy.
Objectives: to establish safety, determine maximum tolerated dose and lay the groundwork for Phase II to assess efficacy.
- Prof. Francine Behar-Cohen, pioneer in ocular electro-transfection and scientific founder of PulseSight, stresses the urgency of a therapy for advanced dry AMD: "We have no therapeutic options for these patients [...] PST-611 could become a great treatment option".
- Judith Greciet, CEO of PulseSight, adds: "We believe PST-611 can improve the anatomical and functional aspects of the disease, reducing the need for frequent reinjections and improving adherence".
Other advanced therapies are already being investigated in Spain:
- The IMO Miranza Group in Barcelona participated in the HORIZON trial with a gene therapy by vitrectomy, led by Dr. Rafael Navarro.
- Other groups, such as Astellas and Ocugen, are exploring gene and cell therapies in geographic atrophy.
- National leaders such as Dr. Jordi Monés, Director of the Institut de la Màcula, promote international trials against macular degeneration.
- Pharmacist and researcher Celia Sánchez-Ramos leads innovation in retinal protection and neuroprotection, crucial for these developments.
If Phase I confirms safety and optimal dose, PulseSight will initiate a Phase IIa in late 2026, seeking to demonstrate the real benefit of PST-611 in preserving retinal structure and function.
The PST-611 trial represents a scientific and clinical breakthrough in the approach to dry AMD and geographic atrophy. If it delivers on its promise, non-viral gene therapy with electro-transfection could transform the therapeutic landscape for millions of people with irreversible vision loss.